Medical Sterilization
First irradiation facility of its kind in the middle east
SureBeam Middle East established the first irradiation facility of its kind in the middle east that uses E-Beam for sterilization of medical devices and disposables. The electron beam, a concentrated highly charged stream of electrons, is generated by equipment referred to as accelerators witch are capable of producing beams that are either pulsed or continuous.
As the products/materials being sterilized passes beneath or in front of the electron beam, energy from the electrons is absorbed. This absorption of energy alters various chemical and biological bonds within the product/materials. It is the absorption of the energy-or "dose deliver"- that destroys the reproductive cells of microorganisms by destroying their DNA chains.
E-Beam radiation is similar to GAMMA processing in that upon contact with the exposed product, electrons alters various chemical and molecular bond-leading to sterilization of the products.
Advanced electronics precisely control the use of electron beams in the sterilization of medical devices. for many materials, E-Beam processing results in less material degradation than with GAMMA irradiation.
SureBeam Middle East electron beam sterilization (E-Beam sterilization) is a commercially successful technology for sterilizing a variety of disposable medical devices with a wide range of densities.
For SureBeam Middle East, meeting your sterilization needs is not simply about providing sterilization for your product. it's about delivering it faster, smarter and less expensively. compared to ever increasing costs by GAMMA or EtO, SureBeam Middle East sterilization services deliver sterilization doses in just few seconds. As a result, the entire sterilization process typically takes minutes or hours rather than several days or weeks. The high put rates means:
Time Advantage
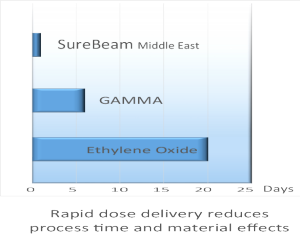
E-Beam processing has the shortest process cycle of any currently recognized sterilization method. In E-Beam processing, products are scanned for seconds, with the bulk of the processing time consumed in transporting products into and out of the radiation shielding. Overall process time, including transport time, is 5 to 7 minutes. Using established and recognized dosimetric release procedures, the product can be released from quarantine within 30 minutes. Another processing time reduction from results from electron beam's ability to change over from one lot to another quickly, thus saving additional time. Products are sterilized as they become available from the upstream production cycle, and no dose groupings or product staging is necessary. For example, E-Beam can change from a product requiring a 25 kGy dose to a different product requiring an 18 kGy dose in the same 5 to 7 minute time frame. As the sterilization process is reduced from weeks or days to hours, inventory working capital requirements are reduced proportionaly.


Food and Drug Administration FDA has approved irradiation of meat and poultry



Dosimetric Release = Immediate Product Release

It is possible to release E-Beam-Processed product immediately upon certification that the process was completed in conformance to specifications, without the need for conventional sterility testing, this release mechanism, known as DOSIMETRIC RELEASE, is based solely on the dosage of radiation delivered to the product and is accepted by the FDA (Food and Drugs Administration).
A description and outline of the process can be found in the American National Standards, ANSI/AAMI/ISO 11137-1994.
SureBeam Middle East Quality System

Safety and Reliability are always of parameters importance. Our ISO 9001 accreditation is underpinned on our strictly observed quality procedures managed by our highly skilled technical staff. SureBeam Middle East certificate is internationally accepted and our sterilization services are carried out in strict conformance to ANSI/AAMI/ISO 11137-1994 standards.
E-Beam Sterilization Advantages
•E-Beam sterilization of medical materials and devices have been in operation internationally since 1950s. •E-Beam sterilization destroys all pathogens (viruses, bacteria, fungi, molds and spores). •E-Beam very efficiently sterilizes target materials with a fast cycle of 2 minutes. It's a low temperature dry process that can be used for most medical materials. An out gassing period is not required. •E-beam sterilization process leaves no toxic residues like EtO. unlike GAMMA radiation E-Beam system uses no hazardous radioactive materials, beside there is no residual radiation when the system is switched off. It produces no toxic or radioactive bye-products.
Comparison
| Key Consideration | Gamma Radiation |
Electron Beam E-Beam |
Ethylene Oxide EtO |
| Process Methodology | Continuous or batch | Continuous | Batch |
| Product Release | Dosimetric release (immediate, no post-sterilization testing is required) | Dosimetric release (immediate, no post-sterilization testing is required) | History, Bls were required to verify sterility assurance level (SAL) |
| Penetration | Complete penetration | Complete penetration, Dependant upon material thickness. | Complete penetration with the use of gas-permeable packaging. |
| Material Compatibility | Most materials are satisfactory. Considered somewhat incompatible with PVC, PTFF and Acetal. | Most materials are satisfactory | nearly all materials are compatible |
| Residuals | None | None | Ethylene Chlorohydrin, requiring period following processing. |




Connect with us
+966 (11) 4984462
faq@smebeam.com